Vaccines for Infectious Disease Indications Additional copies of this guidance are available from the Office of Communication Outreach and Development OCOD HFM-40 1401 Rockville Pike Suite. In the new guidelines posted on its website the FDA said vaccine makers should follow trial participants for at least two months to rule out any major side effects before seeking emergency approval.
 Fda Circular No 2020 036 Guidelines On The Issuance Of Emergency Use Authorization For Drugs And Vaccines For Covid 19 Food And Drug Administration
Fda Circular No 2020 036 Guidelines On The Issuance Of Emergency Use Authorization For Drugs And Vaccines For Covid 19 Food And Drug Administration
In this guidance we FDA or the Agency provide information to assist sponsors in developing vaccines to protect against global infectious diseases.

Fda guidelines for vaccines. The guidance will focus on development and. FDA releases new coronavirus vaccine guidelin. 91 Zeilen The FDA published a guidance document to facilitate the timely.
The FDAs expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age is a significant step in the fight against. Prevention February 2021 Emergency Use Authorization for Vaccines to Prevent COVID-19 February 2021 and others. The FDA on Tuesday released final guidance laying out its standards for approving coronavirus vaccines requiring that any vaccine candidate be at least 50 percent.
The guidelines are applicable for both vaccines in development as well as those which have already received emergency use authorization EUAfrom the. And more than 2665 million have been administered according to the CDC. The new FDA guidelines call for ongoing follow-up of people enrolled in the COVID-19 vaccine trials to look for additional adverse events.
FDA Review of Vaccine Labeling Requirements for Warnings Use Instructions and Precautionary Information. Developing Drugs and Biological Products for Treatment or. An update to the FDAs guidance on COVID-19 vaccine Emergency Use Authorization EUA published in October 2020 provides recommendations for vaccine developers who already have EUA for their COVID-19 vaccines and are seeking to amend their EUA to address new variants.
The FDA guidance is intended to complement other COVID-19 guidances from the FDA such as COVID-19. The FDA guidance is intended to complement other COVID-19 guidances from the FDA such as COVID-19. Master protocol trials are quite complex necessitating a high degree of up-front planning and.
Vaccines as with all products regulated by FDA undergo a rigorous review of laboratory and clinical data to ensure the safety efficacy purity and potency of. Guidance for Industry. The FDA determines when a vaccine is FDA approved While the FDA determines if a vaccine qualifies for FDA approval and sets nonbinding guidelines for manufacturers to follow its not entirely up to the organization to decide when.
That timeline is first determined by the manufacturers of the products seeking FDA approval. Developing Drugs and Biological Products for Treatment or Prevention February 2021 Emergency Use Authorization for Vaccines to Prevent COVID-19 February 2021 and others. More than 339 million vaccine doses have been distributed in the US.
FDA is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of COVID-19. 0206 The Food and Drug Administration has released its long-awaited guidance on how it will issue. According to the FDA guidance studies to assess the effectiveness of a Covid-19 vaccines primary shot and booster dose should compare the immune response induced by the modified version of the.
Nearly 119 million Americans have been fully. The guidelines blocked by the White House are a type of nonbinding document that the FDA routinely uses to advise companies on research and regulatory.
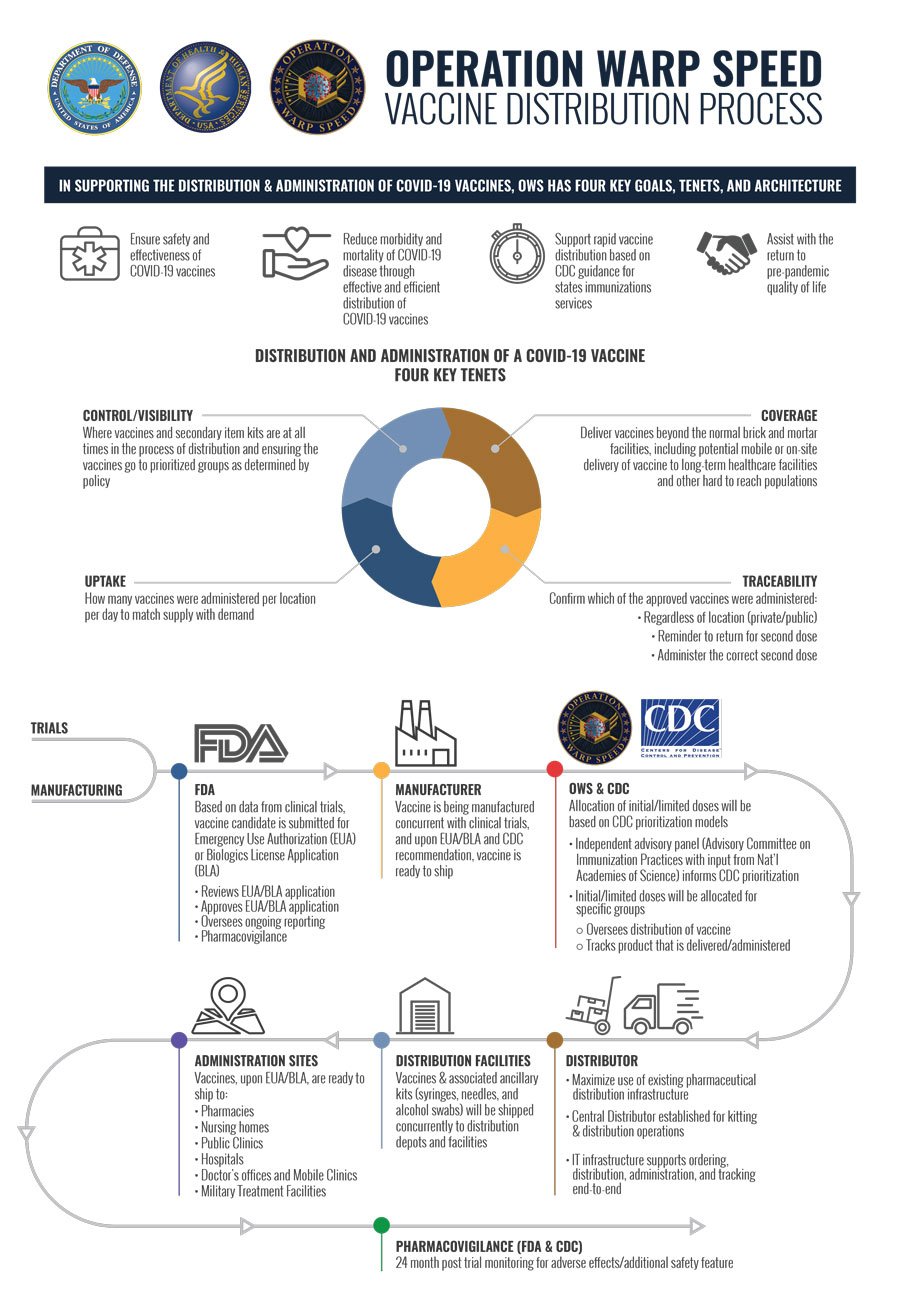 Coronavirus Operation Warp Speed
Coronavirus Operation Warp Speed
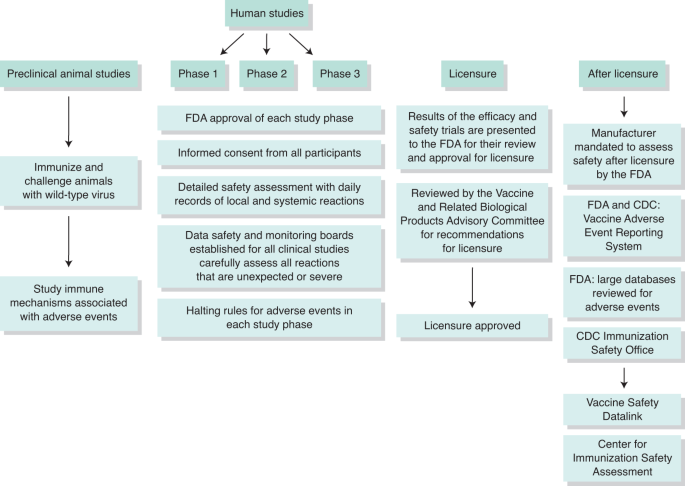 Vaccines Targeting Sars Cov 2 Tested In Humans Nature Medicine
Vaccines Targeting Sars Cov 2 Tested In Humans Nature Medicine
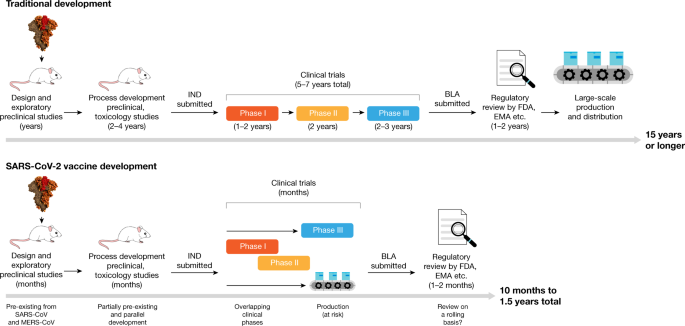 Sars Cov 2 Vaccines In Development Nature
Sars Cov 2 Vaccines In Development Nature
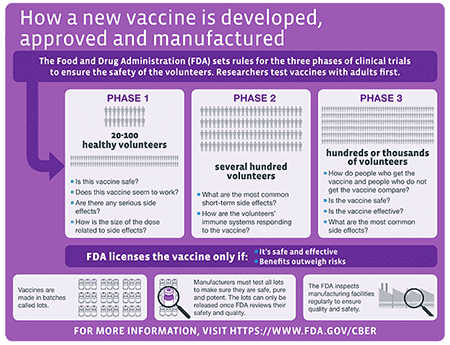 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
 Fda Issues Covid 19 Vaccine Guidance As White House Reportedly Relents Medcity News
Fda Issues Covid 19 Vaccine Guidance As White House Reportedly Relents Medcity News
 Food And Drug Administration Regulation And Evaluation Of Vaccines American Academy Of Pediatrics
Food And Drug Administration Regulation And Evaluation Of Vaccines American Academy Of Pediatrics
Https Www Fda Gov Files Vaccines 20blood 20 20biologics Published Guidance For Industry General Principles For The Development Of Vaccines To Protect Against Global Infectious Diseases Pdf
Https Www Fda Gov Files Vaccines 20blood 20 20biologics Published Guidance For Industry Considerations For Developmental Toxicity Studies For Preventive And Therapeutic Vaccines For Infectious Disease Indications Pdf
 Learn More About Covid 19 Vaccines From The Fda Fda
Learn More About Covid 19 Vaccines From The Fda Fda
 Fda Publishes Vaccine Guidelines Opposed By White House Health News Us News
Fda Publishes Vaccine Guidelines Opposed By White House Health News Us News
 Vaccine Distribution Alabama Department Of Public Health Adph
Vaccine Distribution Alabama Department Of Public Health Adph
 Ama Webinar Vaccine Safety And Delivery Youtube
Ama Webinar Vaccine Safety And Delivery Youtube


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.